Battery
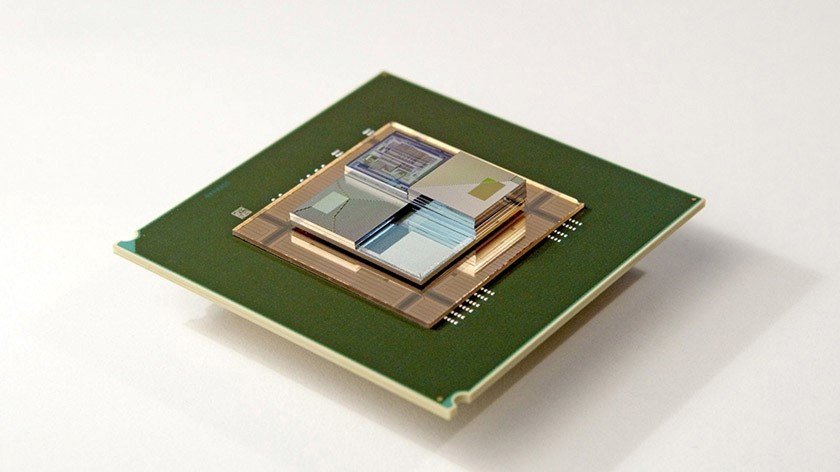
A joint team of researchers from IBM Research Zurich and ETH Zurich laboratory (Swiss Higher Technical School of Zurich) has created a new type of liquid battery. According to the developers, these miniature elements can serve simultaneously as a source of energy and heat sink for the hottest components of electronic devices. Novelty suggests the possibility of a dense vertical placement of chips on the boards to save space and energy.
This battery uses two electrolytes which circulate inside the closed system in a closed circuit. “Chips effectively cool and get the energy necessary for work,” said Dimos Poulikakos, professor of thermodynamics from ETH Zurich.
The battery created by scientists is very thin. Its thickness is only 1.5 millimeters. Its parameters allow to fully realize the idea of developers, which consists in creating a multi-layer “sandwich” of batteries and chips. First, a chip is installed, then a battery, then again a chip and so on.
Liquid batteries for various kinds of devices – not a novelty, they exist. But, basically, they are used as a storehouse of energy in wind and power plants. Here they temporarily store energy in order to later send it to the network. “We are the first who could build such a thin battery, which at the same time is able to supply energy to the circuit board and cool it,” says one of the authors of the project. A battery of this type can both convert the energy of chemical reactions to electricity, and vice versa.
The power of the element is quite good: 1.4 W per square centimeter of area. This should be enough to provide energy for small mobile devices. Test tests have already been carried out, which proved that electrolytic liquids are really capable of cooling the chip.
According to the developers, the main difficulty in creating such batteries was to make them suitable for use with modern electronics. Another difficulty is to reduce the energy consumption of the circulating fluid circuit. The movement of electrolytes requires energy, and if such an element would consume too much energy, it would simply not be suitable as a battery.

Electrochemical reactions in the battery occur due to the presence of two thin porous electrodes, which are separated by a membrane. To create batteries, scientists used 3D printing technology. This was necessary in order to create a polymer system of channels through which electrolytes circulate.
So far, scientists have been able to prove only the potential performance of a liquid battery-cooler. The fact is that he was able to simultaneously cool equipment and provide individual elements with energy. But for modern chips this energy is still too little – the system needs to be improved. Nevertheless, experts are confident of success. In addition, the liquid battery maintains the temperature of the entire system at the optimum level.
The technology was named Redoxflow.









